ONC-392/BNT-316(Gotistobart) – a New Promising Drug for Cancer Patients
WRITTEN BY SHUBO ZHANG
ILLUSTRATED BY CHARLOTTE CHANG
Glossary
- NSCLC: Most common lung cancer, leading to uncontrolled growth of cells in the lungs
- ONC-392/BNT-316/Gotistobart: The main drug discussed; all names refer to the same drug throughout the testing phases.
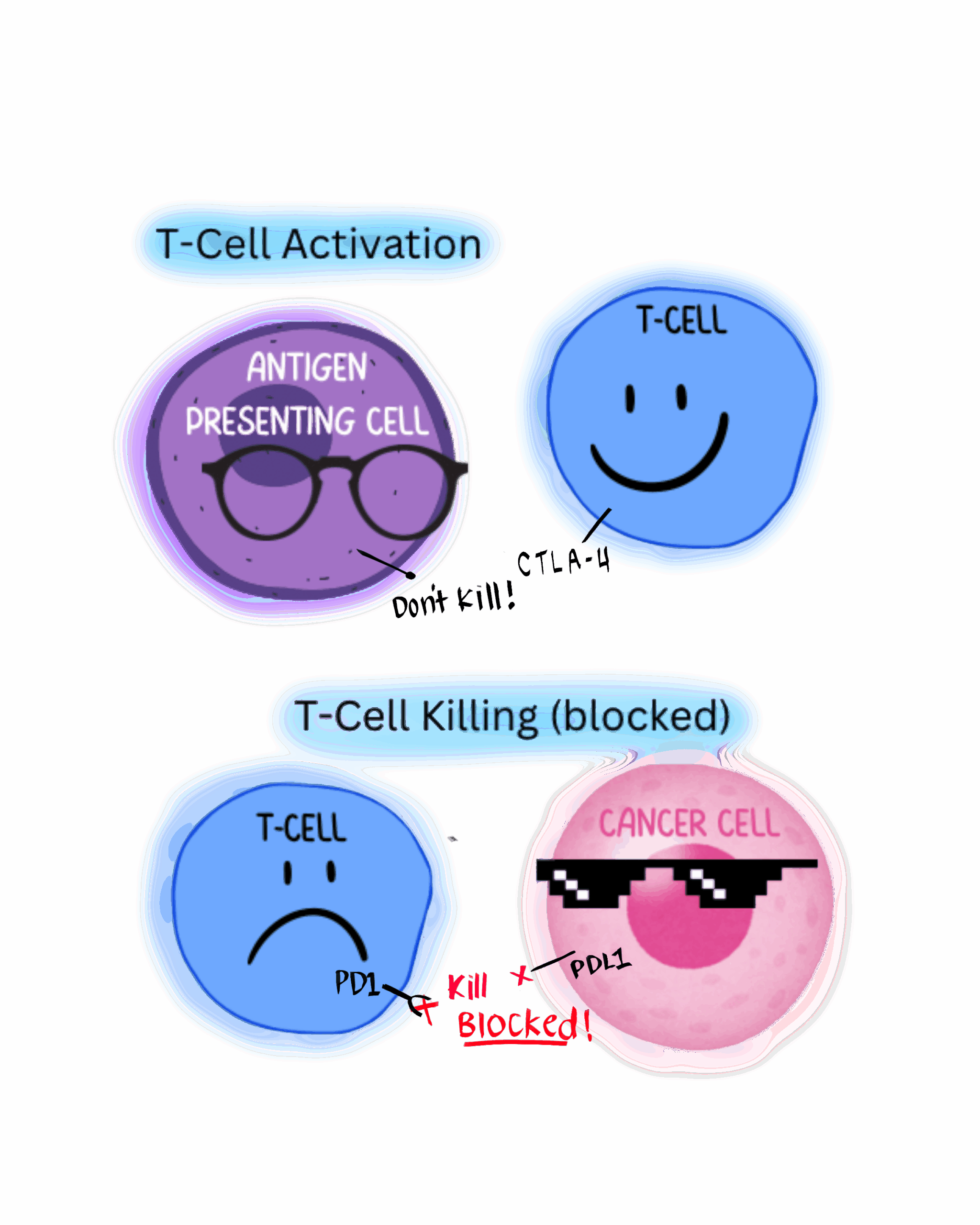
- CTLA-4: Cytotoxic T-lymphocyte antigen 4; an immune checkpoint that restricts T-cell activity and fights against immune response.
- PD-L1: Programmed death ligand that, when bound, inhibits T-Cell activity. A more commonly targeted protein in cancer therapies.
- PD-1: The receptor on the immune cell that binds to PD-L1 in order to be inhibited
- IrAE: Immune-related adverse events; complications that impact the patient’s condition as a result of the therapy
- Ipilimumab, TremelgG1, and AB157: Therapies that target CTLA-4 proteins
Abstract
This review presents current literature and most recent publications on a new drug called ONC-392/BNT-316, also known as Gotistobart, a promising new treatment for cancer. Currently, there are ways to manage and treat non-small cell lung cancer (NSCLC), but due to drug resistance, cancer type, and tumor targeting, cancer as a disease is yet to be fully addressed. This article reviews the unique method underlying the drug’s mechanistic pathways, potential implications, and the impact moving forward.
Background
Cancer’s ability to deceive the body and disguise itself as a normal cell makes it one of the most difficult diseases to treat. Normally, processes exist to terminate the cell at a given moment. Cancer’s self-preservation mechanisms work by stopping these terminals for signaling cell death. An important ability of the immune system are auto-immune attacks. Cancers that persist in the body are often able to ignore the signal through artificially using immune-suppressing checkpoints. By hijacking these checkpoints, the cancer cell can escape the attack mounted by the immune system.
One such checkpoint is the cytotoxic T-lymphocyte antigen four (CTLA-4) checkpoint, a signal protein found in T-regulatory cells that binds to killer T-cells and other immune cells to deactivate the immune response. Another is PD-L1, a protein that signals cell death, which has been traditionally targeted in combination with chemotherapy, the most common treatment cancer patients receive. These treatments use pathways such as immune checkpoints present on cells and cancers as an identifier to attack them.
However, higher doses of these treatments may trigger excessive immune responses. Such responses manifest in symptoms of immune-related adverse events (irAE). IrAEs are common complications in conditions that result from cancer treatment targeting immune checkpoint inhibitors like CTLA-4. Drugs with anti-CTLA-4 function prevent T-regulatory cells from stopping anti-cancer activity and inhibit T-cell regulation around the body. As a result, these drugs prevent T-cell regulator function and allow T-cells to indiscriminately attack all cells. This unregulated immune activity leads to tissue damage and inflammatory disease in organs like the eyes and skin.
In the study conducted by Dr. Xuexiang Du and Dr. Mingyue Liu on mice, investigators compared the current market standard drug, Ipilimumab (a CTLA-4 inhibitor), with PD-1 (another immune checkpoint protein) blockers. They’ve shown that Ipilimumab displays cytotoxic activity (irAE) of grade 3-4, which indicate dangerous levels of toxicity that may compromise patient condition.1
Preclinical
Before worrying about toxicity within patients, researchers must demonstrate their concept in a controlled environment like a mouse. In the mouse model, investigators inserted a human cancer cell to imitate the tumor microenvironment. They then treated groups of mice with Ipilimumab, TremelgG1, AB157, and other marketed antibodies and candidates for CTLA-4 blocking. Investigators measured the binding efficiency, T-regulatory cell depletion, irAE response, and tumor control of various conditions and found a promising new protein excelling in all three: Gostibobart.
pH sensitive drugs on the market (like Iipilimumab) that attach to the CTLA-4 indefinitely cause the cell to degrade both the antibody and the attached protein. In a study of Ipilimumab, they found that when interacting with CTLA-4, the drug is actually absorbed and taken into the cell to the lysosome, where it mixes with the anti-CTLA-4 antibody. Since lysosome digests proteins, they both would be degraded, and the cell loses the CTLA-4 pathway used to prevent irregular T-cell activity.
Gotistobart differs from other lab-made proteins. Whereas current CTLA-4-targeting drugs bind more permanently to T-regulatory cells, inhibiting them even when the cancer is gone, Gotistobart preserves the function of the CTLA-4 on T- regulatory cells by allowing the antibody to be reused rather than being degraded through the lysosome in a pH-sensitive interaction within the cell.2 As the bound CTLA-4 receptor moves from high pH blood into lower pH blood, antibodies unbind and are recycled into the membrane. In the long term, the salvaged receptor can continue to regulate T-Cell activity after the treatment finishes. Gotistobart also detaches once the therapeutic effect has occurred. In this way, only the drug protein is degraded, which leaves the CTLA-4 antibody to re-express on the surface of the cell and theoretically allows for a higher limiting dosage due to decreased irAE sensitivity.1
Phase I: Finding Optimal Dosage and Drug Stability
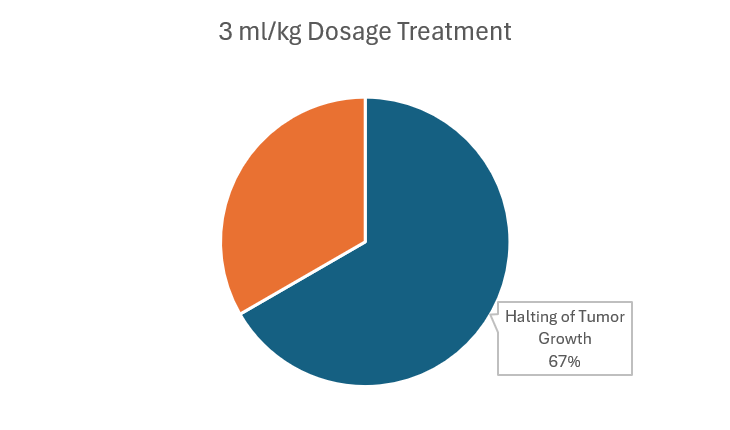
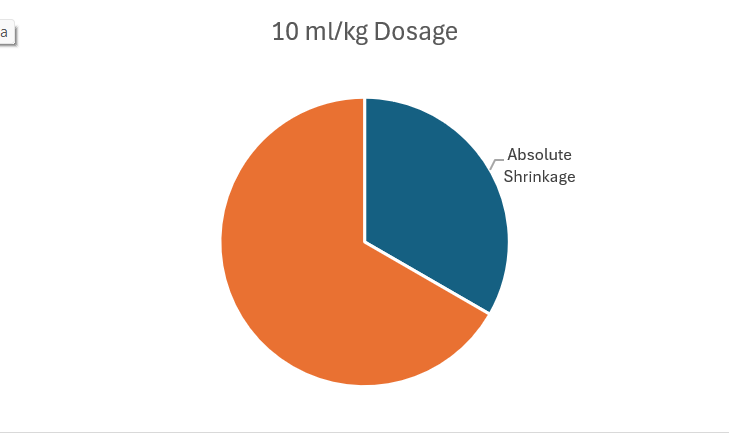
In this stage, multiple non-small cell lung cancers (NSCLC) and metastatic solid tumors were tested for dosage of drug stability.7 Typically, Phase I trials will use data from mice and other treatments as a starting point for minimum treatment. Investigators begin at 3 ml/kg and escalate with three patients at a time until roughly one-third of the patients experience irAE to determine the dosage that maximizes effectiveness and minimizes damage.
While other drugs like Ipilimumab are capped at 3 mL/kg, limiting their effects, Gotistobart can go up to 10 kg/mL with greater efficiency and less adverse effects. The investigators also found that it was best to limit the 10 mL/kg up to three to four doses to maximize efficiency without excessive risk of irAEs. Within multiple treatments of 3 ml/kg, four of the six of the patients showed halted tumor growth. Even with 10 ml/kg, two out of six patients saw a shrinkage of the tumor. This preclinical data already shows promise of being more effective and safe than current market drugs.8
Phase II: Drug Efficacy and Performance Relative to Market Standard
Phase II deals with the drug’s compatibility with current market standard CTLA-4 and PD-1 drugs to test the potential for combination treatment with others on the market. For example, injecting each drug into a different arm to look for both the instant reaction and long term control would demonstrate combination effects that can be monitored over time. Proceeding tests explore combination treatment in comparison to Gotistobart alone in specific patients to determine cancer type and how many cancer types the drug is effective for.
Cancers with NSCLC and PD-L1 resistance are currently a major issue within cancer-treating drugs. If researchers are able to achieve success in these environments, it would mean Gotistobart is uniquely able to treat patients that are otherwise unaffected by treatment. As the trial progresses, researchers expect the drug will be tested for efficacy in other types of cancers and with other drug combinations.
Phase III: Testing Specific Conditions in a Larger Demographic
After demonstrating success in treatment-resistant NSCLC, investigators are moving forward with a clinical trial of about 600 people across the U.S., Europe, and other countries with late-stage melanoma NSCLCs in hopes of using survival and control rate as a marker for success. Development of Gotistobart in collaboration with various biotechnology companies will continue as both monotherapy and combination therapy in a variety of treatment resistant cancers.15
In addition, researchers tested the potency of Gotistobart to induce the rejections of large tumors by the body, specifically NSCLC that are PD-1 resistant.14 These PD-1 resistant tumors are currently a large factor in successful treatment, and as the trials continue, their impact on survival may decrease. Investigators are hopeful that stopping T-cell regulation may boost the effect of current treatment options like chemotherapy.
Results:
The two main issues with CTLA-4 persist in market standard treatments like Ipilimumab: cytotoxicity and efficacy. With PD-1 inhibitors serving as a predecessor pathway for Gotistobart, the drug Gotistobart represents an alternative and a massive breakthrough. As a CTLA-4 inhibitor, it is both less toxic and more effective than drugs in the current market.4 Gotistobart was found to be more potent at a lower concentration in the tumor microenvironment compared to Ipilimumab, which was found to block only 25% of CTLA-4 binding and less than 50% at higher concentrations.5
Discussion:
Resistance to current methods of cell death (PD-1) inhibitors demand new solutions. This new CTLA-4 pathway provides an alternative route to recovery for patients with or without the current PD-1 drugs. Its potential goes beyond a potential treatment, but may also serve as a supplement to work in coordination with current treatments, such as platinum based drugs like Cisplatin, Carboplatin, and Oxaliplatin that bind to DNA in cancer cells and stop them from replicating.9 This is especially impactful in platinum-resistant ovarian cancer, where 40% of diagnosed patients die within 1 year of diagnosis and 25% within 90 days of diagnosis.10 These conditions prove exceptionally problematic for patients because the most common method of treatment, chemotherapy, is often administered with these platinum-based drugs.11
As of now, lung cancer is the most common cancer, making up 11.6 percent of all cancers and the leading cause of cancer death (18.4 percent of cancer deaths), which leads to 2 million cases every year and 1.76 million deaths.12 NSCLC make up about 85% of lung cancers yet have a five year survival rate of about less than 25%.13 With Gotistobart, we can expect data that shows an increase in five year survival rate in NSCLC patients and PD-1 resistant patients.
Conclusion:
Gotistobart is a new drug developed from cloning an antibody that exists within the body that targets the CTLA-4 that regulates and restricts T-Cell activation. Unlike the current PD-1 treatments, this indirectly kills the cancer by increasing T-Cell activity and provides alternatives for those resistant to current treatment methods.
The introduction of Gotistobart represents a groundbreaking advancement in cancer treatment, offering a promising alternative to existing therapies, particularly for patients resistant to current PD-1 or platinum-based treatments. Through its innovative mechanism of recycling antibodies while preserving the functionality of CTLA-4 on T-regulatory cells, Gotistobart has demonstrated superior efficacy and reduced toxicity compared to other monoclonal antibodies like Ipilimumab. This unique approach allows for higher doses with fewer immune-related adverse events, a key consideration in optimizing treatment for non-small cell lung cancer (NSCLC) and other cancers.
The drug’s potential to address treatment-resistant cancers may prove crucial in managing current challenges in these conditions. By enhancing immune response without triggering excessive immune reactions, Gotistobart not only opens the door for more effective monotherapy but also shows promise as a complementary treatment in combination with existing therapies, amplifying the potential for improved patient outcomes.
As Gotistobart moves into phase III trials, its continued success in clinical studies could lead to broader applications across various cancer types, ultimately transforming the landscape of cancer treatment. This development marks a pivotal step toward overcoming the limitations of current therapies, offering hope for improved survival rates and better quality of life for patients facing some of the most aggressive and resistant forms of cancer. The future of Gotistobart is bright, with the potential to reshape the standard of care for patients worldwide, particularly those who have limited treatment options.
References
- Du, X.; Liu, M.; Su, J.; Zhang, P.; Tang, F.; Ye, P.; Devenport, M.; Wang, X.; Zhang, Y.; Liu, Y.; Zheng, P. Uncoupling Therapeutic from Immunotherapy-Related Adverse Effects for Safer and Effective Anti-CTLA-4 Antibodies in CTLA4 Humanized Mice. Cell Research 2018, 28 (4), 433–447. https://doi.org/10.1038/s41422-018-0012-z.
- BioNTech and OncoC4 Present Positive Phase 1/2 Data for Antibody Candidate BNT316/ONC-392 in Hard-to-Treat NSCLC at ASCO | OncoC4. Oncoc4.com. https://oncoc4.com/press-releases/biontech-and-oncoc4-present-positive-phase-1-2-data-for-antibody-candidate-bnt316-onc-392-in-hard-to-treat-nsclc-at-asco/ (accessed 2025-03-27).
- Thai, A. A.; Solomon, B. J.; Sequist, L. V.; Gainor, J. F.; Heist, R. S. Lung Cancer. The Lancet 2021, 398 (10299), 535–554. https://doi.org/10.1016/S0140-6736(21)00312-3.
- He, K.; Carbone, D. P.; McKean, M.; Balaraman, R.; Shah, S.; Arrowsmith, E.; Peguero, J. A.; Joshi, R.; He, A. R.; Milillo, A.; Hamm, J. T.; Goldstein, M. G.; Li, Z.; Liu, Y.; Zheng, P.; Li, T. Safety and Clinical Activity of Target-Preserving Anti-CTLA-4 Antibody ONC-392 as Monotherapy in NSCLC Patients Who Progressed on PD(L)1-Targeted Immunotherapy. Journal of Clinical Oncology 2023, 41 (16_suppl), 9024–9024. https://doi.org/10.1200/jco.2023.41.16_suppl.9024.
- Du, X.; Tang, F.; Liu, M.; Su, J.; Zhang, Y.; Wu, W.; Devenport, M.; Lazarski, C. A.; Zhang, P.; Wang, X.; Ye, P.; Wang, C.; Hwang, E.; Zhu, T.; Xu, T.; Zheng, P.; Liu, Y. A Reappraisal of CTLA-4 Checkpoint Blockade in Cancer Immunotherapy. Cell Research 2018, 28 (4), 416–432. https://doi.org/10.1038/s41422-018-0011-0.
- National Institute on Aging. What Are Clinical Trials and Studies? National Institute on Aging. https://www.nia.nih.gov/health/clinical-trials-and-studies/what-are-clinical-trials-and-studies.
- Rolfo, C. D.; Bentzen, S.; Devenport, M.; Liu, Y.; Zheng, P. First-In-Human Phase I/II Clinical Trial of ONC-392: Preserving CTLA-4 Immune Tolerance Checkpoint for Safer and More Effective Cancer Immunotherapy. Journal of Clinical Oncology 2020, 38 (15_suppl), TPS3159–TPS3159. https://doi.org/10.1200/jco.2020.38.15_suppl.tps3159.
- Rolfo, C. D.; Bentzen, S.; Devenport, M.; Liu, Y.; Zheng, P. First-In-Human Phase I/II Clinical Trial of ONC-392: Preserving CTLA-4 Immune Tolerance Checkpoint for Safer and More Effective Cancer Immunotherapy. Journal of Clinical Oncology 2020, 38 (15_suppl), TPS3159–TPS3159. https://doi.org/10.1200/jco.2020.38.15_suppl.tps3159.
- Zhang, C.; Xu, C.; Gao, X.; Yao, Q. Platinum-Based Drugs for Cancer Therapy and Anti-Tumor Strategies. Theranostics 2022, 12 (5), 2115–2132. https://doi.org/10.7150/thno.69424.
- Urban, R. R.; He, H.; Alfonso, R.; Hardesty, M. M.; Gray, H. J.; Goff, B. A. Ovarian Cancer Outcomes: Predictors of Early Death. Gynecologic Oncology 2016, 140 (3), 474–480. https://doi.org/10.1016/j.ygyno.2015.12.021.
- National Cancer institute. Welcome to SEER Training | SEER Training. Cancer.gov. https://training.seer.cancer.gov/.
- Zhang, Y.; Du, X.; Liu, M.; Tang, F.; Zhang, P.; Ai, C.; Fields, J. K.; Sundberg, E. J.; Latinovic, O. S.; Devenport, M.; Zheng, P.; Liu, Y. Hijacking Antibody-Induced CTLA-4 Lysosomal Degradation for Safer and More Effective Cancer Immunotherapy. Cell Research 2019, 29 (8), 609–627. https://doi.org/10.1038/s41422-019-0184-1.
- BioNTech and OncoC4 Present Positive Phase 1/2 Data for Antibody Candidate BNT316/ONC-392 in Hard-to-Treat NSCLC at ASCO | OncoC4. Oncoc4.com. https://oncoc4.com/press-releases/biontech-and-oncoc4-present-positive-phase-1-2-data-for-antibody-candidate-bnt316-onc-392-in-hard-to-treat-nsclc-at-asco/.
- Clinicaltrials.gov. https://clinicaltrials.gov/study/NCT05446298?intr=ONC-392&limit=25&page=1&rank=1 (accessed 2025-03-27).
- BioNTech and OncoC4 Initiate Pivotal Phase 3 Trial of BNT316/ONC-392 Program in Metastatic NSCLC | OncoC4. Oncoc4.com. https://oncoc4.com/press-releases/biontech-and-oncoc4-initiate-pivotal-phase-3-trial-of-bnt316-onc-392-program-in-metastatic-nsclc/.
