Bleach: A Double-Edged Sword for Cleaning
WRITTEN BY JAGGER NELSON
ILLUSTRATED BY ANDREA ARMENDI
In 2019, a Buffalo Wild Wings employee collapsed and later died after mopping the floor with a bleach-based cleaner that had been accidentally mixed with an acidic solution.1 He had simply been doing his job, unaware that combining two common products would release chlorine gas—a toxic chemical weapon once used in World War I.
During the early months of the COVID-19 pandemic, poison control centers across the U.S. and Canada saw a sharp increase in emergency calls linked to household cleaners.2 One woman, attempting to sanitize her produce, filled her sink with bleach and vinegar, a combination she believed would protect her family. Instead, she was rushed to the ER after inhaling chlorine fumes.3
These are not isolated incidents, but symptoms of a widespread problem. Cleaning is often seen as routine and harmless, but most people don’t realize that bleach, when mixed with certain chemicals, can become dangerous—even deadly. Without a basic understanding of the chemistry behind household products, even well-intentioned actions can result in hospitalization, long-term lung damage, or worse.
This piece isn’t meant to scare you away from bleach, but to arm you with enough knowledge to use it safely. With real-world examples, simple chemical principles, and a checklist you can follow without a science degree, we can reduce accidental poisonings and make homes safer for everyone.
So, what is bleach? It turns out that most household bleaches are composed of 5% sodium hypochlorite (NaOCl). That means that for every 95 grams of water in the solution, there are five grams of the salt NaOCl. Technically, you won’t ever find the “solid” form of NaOCl as a salt because it is very reactive with CO2 in the air, even potentially explosive. Most bleaches are manufactured by sending current through salt water, effectively splitting the Na and Cl ions in solution before joining with NaOH to form aqueous NaOCl.4
Most bleach manufacturers recommend diluting bleach to 1:100 (0.05% w/w). In other words, for every one part bleach, 99 parts of water should be used. In an everyday context, if you wanted to use bleach to clean your house, you would only need one fluid ounce of bleach to a gallon of water for the bleach to be “safe” and effective. However, even the diluted version of bleach is still corrosive, and you should always wear PPE (personal protective equipment such as gloves and a mask) whenever you work with bleach! The danger usually comes from using it straight from the bottle and not knowing how it can affect our health.
Now you might be wondering, how does bleach actually work? Well, bleach denatures the proteins of microbes and damages their DNA/RNA, which is a double-edged sword for humans as well. The OCl– (hypochlorite ion) performs this through oxidation, which is the same process as rusting away a metal but on the blueprints of cells. This is exactly why bleach is used in hospital settings, as it is an extremely effective sterilizing agent. If bleach got on your skin, its highly corrosive nature would start to burn away your tissues. Remember to wash with lots of water and only water if this happens to you!
The two examples of accidental poisonings mentioned in the beginning had something in common: mixing bleach with other chemicals. Specifically, when bleach is mixed with an acid, the pH of the solution becomes significantly reduced, and HOCl (bleach’s conjugate acid) reacts with hydrochloric acid to produce water and chlorine gas. Even when chlorine gas is inhaled at low concentrations, it can irritate the mucous membranes by reacting with the water in your lungs and throat to produce hydrochloric acid, an extremely corrosive acid. If enough chlorine gas is inhaled, it can lead to fluid filling in the lungs and adult respiratory distress syndrome, requiring hospitalization.5
Acids are not the only chemicals that shouldn’t be mixed with bleach, as alcohols and ammonia-related solutions can create chloroform/chloramine gases, which are just as bad as chlorine gas. These gases can cause coughing, chest pain, shortness of breath, and even long-term lung damage with enough exposure. For this reason, it’s critical to read labels and avoid combining household cleaners unless the mixture is explicitly recommended by the manufacturer.
To help you out, I have created a checklist below that summarizes how to use bleach:
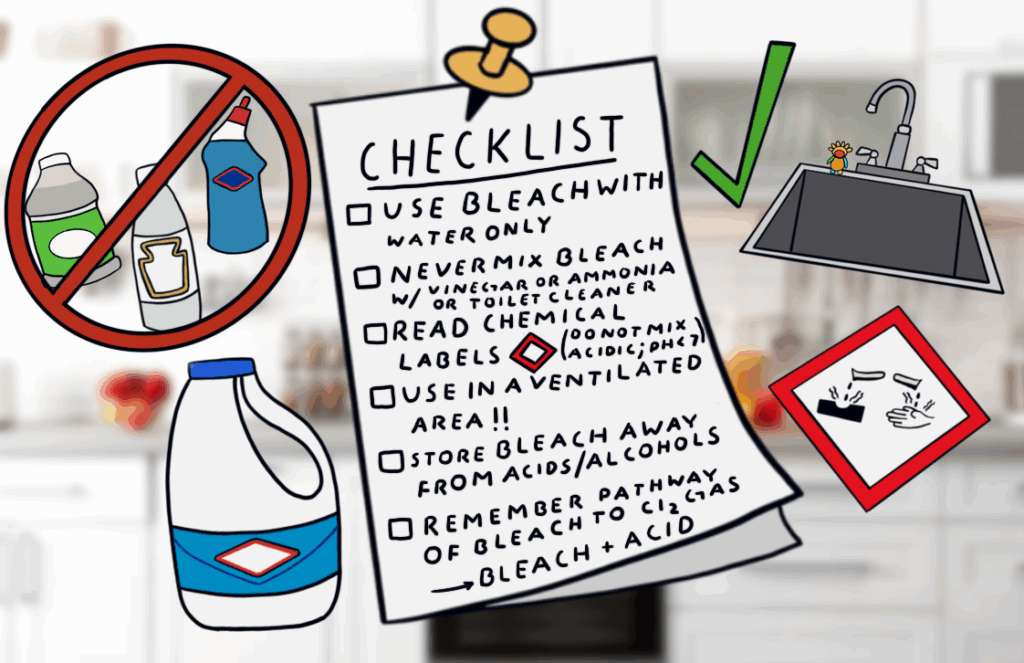
Last but not least, you can always look up the SDS (Safety Data Sheet) for any household chemical with a quick Google search. These can tell you about the hazards and information of any manufactured chemical.
At the end of the day, this isn’t about being scared of cleaning—it’s about being smart. Bleach does its job when used properly, but it’s not meant to be mixed, layered, or experimented with. You don’t need to memorize chemical equations or understand every reaction, but knowing which products to keep separate can save your life or someone else’s. So read the label, keep your acids and bases apart, and stick to the basics: bleach + water, that’s it. The chemistry isn’t going away, but with a little knowledge, the danger can.
References
- Boerner, L. Accidental mix of bleach and acid kills Buffalo Wild Wings employee https://cen.acs.org/safety/consumersafety/Accidental-mix-bleach-acidkills/97/i45 (accessed 2025-04-12).
- Yasseen III, A.; Weiss, D.; Remer, S.; Dobbin, N.; MacNeill, M.; Bogeljic, B.;Leong, D.; Wan, V.; Mosher, L.; Bélair; G.; Thompson, M.; Button, B.; Hardy, J.; Perwaiz, S.; Smith, A.; Wootton, R. Increases in Exposure Calls Related to Selected Cleaners and Disinfectants at the Onset of the COVID-19 Pandemic: Data from Canadian Poison Centres. Health Promotion and Chronic Disease Prevention in Canada 2021, 1 (1), 25–29.
- Kluger, J. As Disinfectant Use Soars to Fight COVID-19, So Do Accidental Poisonings https://time.com/5824316/coronavirus-disinfectant-poisoning/ .
- Gagliardi, M. What Is Bleach and What Are Its Active Ingredients? https://www.clorox.com/learn/what-is-bleach-what-are-active-ingredients/ (accessed 2025-04-13).
- Chlorine gas toxicity – StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK537213/ (accessed 2025-04-12).
