The (Anti) Coagulation of Blood Through Protein Communication
WRITTEN BY JORGE LOZOYA
ILLUSTRATED BY CASSANDRA CHANG
Blood is vital in keeping us alive and healthy. Hemostasis, or the coagulation of blood, is one of the many ways our blood helps by thickening itself to cover injured cells and prevent infection.1
Without hemostasis, bleeding from even the smallest cut would be lethal, which is why there can be drastic consequences should the process of coagulation be interrupted, delayed, or even accelerated via a faulty protein. Therefore, knowing how the human body responds to bleeding wounds can result in methods to not only reduce the lethality of serious injuries, but also enhance medicine, research, and overall quality of life. Through countless years of research, scientists today have found that the proteins that make our blood are the primary means to recover from serious injury.
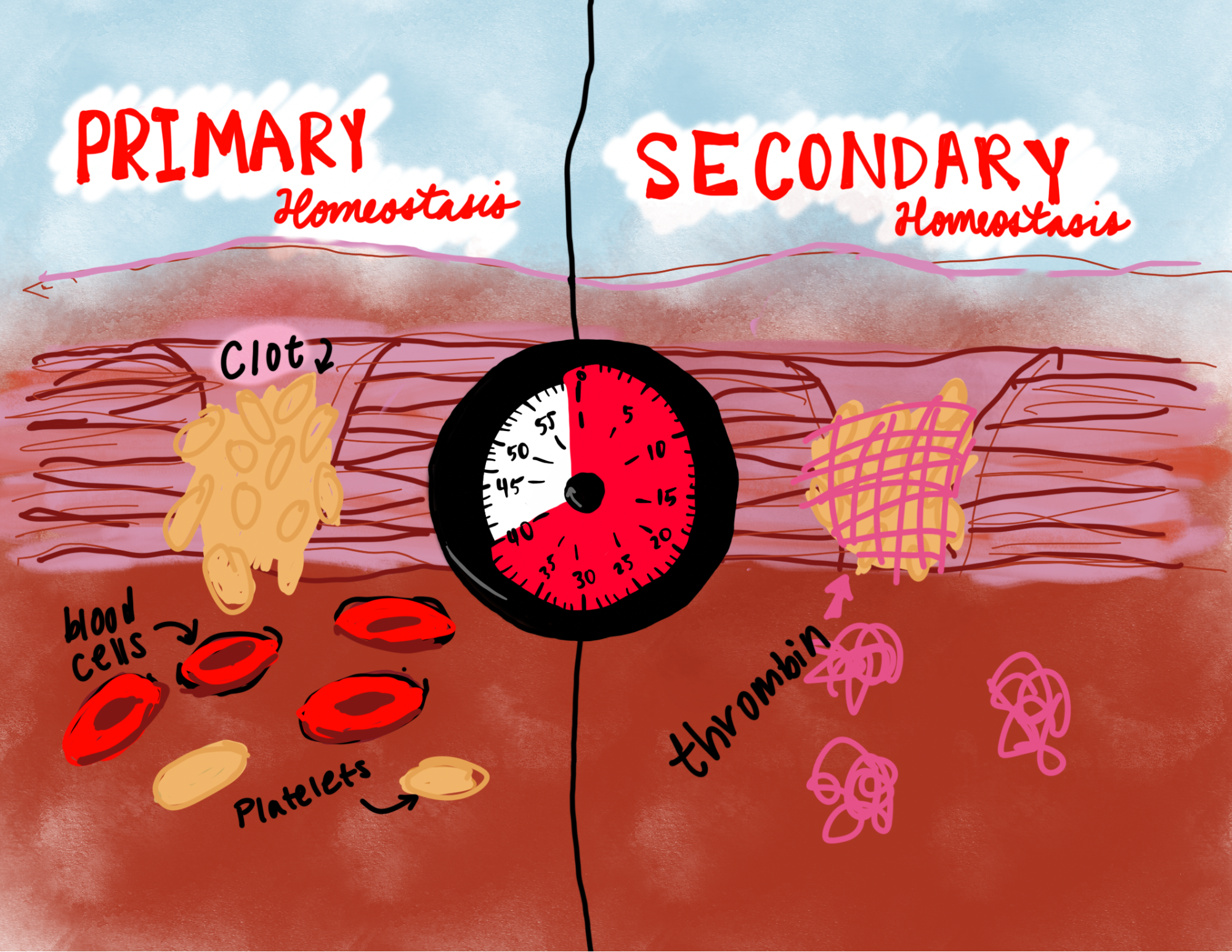
There are two steps that happen during hemostasis. Primary hemostasis starts immediately when the body is cut open. Red blood cells move into the cut’s opening to form a dry covering called a platelet around the wound that prevents more blood from escaping the body and invading agents from getting in. However, this clot is highly unstable as there is nothing chemically binding the blood cells together. Anything as simple as blood flow or a finger scratch will break the platelet’s fragile binding and eventually dissolve it, thus opening the cut again.
Secondary hemostasis, or the coagulation cascade, is the most important factor to making coagulation a possible and reliable choice for survival. The cascade is activated when the body uses vitamin K to release thrombin, a protein that travels to the wound and binds to fibrin proteins found around blood cells. Thrombin is a coagulant and acts as a glue for the clot, chemically binding the blood cells together to make a covering strong enough to properly seal the wound.
Eventually, the wound will need to be replaced with the cellular tissue required to fully heal, and that means removing the blood clot that is tightly bound to the wound.2 Thrombomodulin (TM) is the receptor that finishes the coagulation cascade and heals the wound. TM binds to thrombin and changes the protein into an anticoagulant, or a blood thinner, by reversing the chemical binding performed by thrombin and allowing the clot to be removed by blood flow as soon as cell tissue grows to replace it. This is a slow process that takes anywhere from a few hours to several days, but the result is a body with no significant cuts.2
The coagulation cascade is meant as a reliable way to treat a cut to the body. However, as noted by the immensely complicated and precise steps that go in coagulation, problems can occur with the smallest errors. For example, hemophilia is a bleeding disorder with symptoms of bruising and overly excessive bleeding that happens if the body activates either too much or too little thrombin and TM, if TM cannot successfully transform thrombin into a blood thinner, or if thrombin cannot bind with fibrin.3 Excessive bruising and bleeding can also happen if there is not enough vitamin K in the system, either from the person’s body not processing enough vitamin K or not eating enough greens such as kale.3 Any error to the making or use of the two proteins can lead to the whole cascade failing, leading to a person’s body vulnerable to bleeding and infections.
So how do these proteins communicate with each other? And how do they cooperate so well as to make a system like the coagulation cascade be done so successfully? This is what Dr. Elizabeth A. Komives and her team at UC San Diego are working to investigate as they learn more about thrombin and TM and how they communicate with each other to coagulate and thin blood in a seemingly flawless operation. The team uses several systems to identify how certain proteins interact with each other. One of these is through Dr. Komives’ own service as faculty oversight in the university’s mass spectrometry facility.4 These facilities use special machines to detect changes in the weights and masses of the thrombin and TM proteins at the microscopic scale. Their findings reveal that thrombin and TM have a unique loop shape that can only be modified by each other, meaning the properties of thrombin—and how they can quickly be changed—can only be performed with TM and vice versa. In addition, they concluded that thrombin goes through a process known as an H/D exchange, where the amount of loops the specific thrombin was showing was changed by interacting with a TM protein, thereby changing how solvent it is and changing how it binds to blood and other protein fibers.

This knowledge can be used to create massive progress in modern medicine. In surgeries, thrombin can be used as an agent to stop or enhance bleeding depending on the presence of TM. Using this strategy, surgeons can suppress bleeding practically anywhere in the body, from traumatic injuries to incredibly precise eye surgeries. Where the slightest mistake with the scalpel used to lead to a damaged eye now becomes a simple mistake that can be corrected easily.5
Thrombin usage does not stop at just the medical field. Topical products containing thrombin are being sold so that open wounds close faster than normal when applied. Research is ongoing to create special gels that can heal places that are normally too sensitive, such as cancerous tumors. In addition, knowledge on bleeding prevention can be used to treat lethal damage caused by disorders such as brain aneurysms.
Blood keeps us moving forward, and is able to do an even greater capacity if we learn to better maintain and care for it. Besides at home and at the hospital, there are numerous other ways that coagulation research can better our lives, and discovering new knowledge is the best way for us to lead happier and healthier lives. Findings on blood research is only just beginning, and new methods to improve blood care appear every week. It is time to take a deeper look at the important research done on the blood that is vital in keeping us thriving.
Acknowledgements
I want to give my thanks to the Komives lab for being a great source of information in this article. I found their research at the ACS Research symposium in May of 2024, where I spoke with one of the undergraduate students at their stand. I was very fascinated by what they found with thrombin and TM. Someday, I wish I can join their lab as the research they do has truly caught my attention and I can’t be more excited to see where their research will go in the coming months.
References
- Tarantino, C. Coagulation Cascade: What Is It, Steps, and More | Osmosis. www.osmosis.org. https://www.osmosis.org/answers/coagulation-cascade.
- Peacock, R. B.; McGrann, T.; Zaragoza, S.; Komives, E. A. How Thrombomodulin Enables W215A/E217A Thrombin to Cleave Protein c but Not Fibrinogen. Biochemistry 2022, 61 (2), 77–84. https://doi.org/10.1021/acs.biochem.1c00635.
- Smiley, B. Vitamin K Deficiency: Causes, Symptoms, and Treatment. Healthline. https://www.healthline.com/health/vitamin-k-deficiency#symptoms.
- Elizabeth A. Komives – KomivesLab. Ucsd.edu. https://komiveslab.ucsd.edu/?page_id=88 (accessed 2024-09-12).
- Guo, Y.; Wang, M.; Liu, Q.; Liu, G.; Wang, S.; Li, J. Recent Advances in the Medical Applications of Hemostatic Materials. Theranostics 2023, 13 (1), 161–196. https://doi.org/10.7150/thno.79639.
