A Look at the Chemistry of Einsteinium and other Trans-Plutonium Elements
BY GORDON PEIKER
When a chemist needs a chemical or compound it is usually a trivial task to walk over to the stockroom or order it online. However, when a chemist needs a sample of einsteinium, instead of going to the stockroom they must go to one of the few facilities in the world that can produce it, such as Oak Ridge National Lab. Einsteinium is so labor and resource-intensive to produce that since it was first discovered in the ashes of a nuclear test in 1952 Oak Ridge has only been able to make 40 mg.1,2 Despite these difficulties in production, a recent study in the journal Nature was able to characterize a coordination complex of einsteinium and reveal interesting and novel properties of the element.3
Heavy and super heavy elements such as einsteinium can only be produced by high-powered reactors such as those at Oak Ridge. These reactors accelerate neutrons into collisions with lighter elements such as americium and curium known as the feed-stock. Sometimes, when a collision occurs, an element of the feed-stock will capture a neutron and increase its mass number by one.2 This element with an extra neutron can then undergo beta radiation. If this beta decay happens before another neutron collision happens then the neutron becomes a proton and the element’s atomic number goes up by one, in other words, making a new element.2 This process repeats itself to produce both a range of isotopes and eventually a range of elements including berkelium, californium, einsteinium, and fermium.2 These elements then must go through a complex separation and purification process which includes several months of decay time to eliminate short-lived radioactive products.2
What all of this means is that for Dr. Rebecca Abergel and her team at the University of California Berkeley to have the chance to study einsteinium is a marvel. But even beyond the difficulty in production, there were numerous other challenges. For instance, working with radioactive einsteinium is a race against time. The particular isotope used by Dr. Abergel’s group, einsteinium-254, has a half-life of only 276 days.4 Everything from the separation, purification, and transportation all significantly eat into the amount of einsteinium available. This radioactivity also presents dangers in handling, requiring special novel containers to store the einsteinium.5
Despite all of this the team at Berkeley was still able to learn a significant amount about the largely unexplored chemistry of einsteinium. While X-ray crystallography was considered, ultimately it was decided to explore the chemistry of einsteinium in relation to the ligand 3,4,3-LI(1,2-HOPO) which is pictured below in Figure 1.
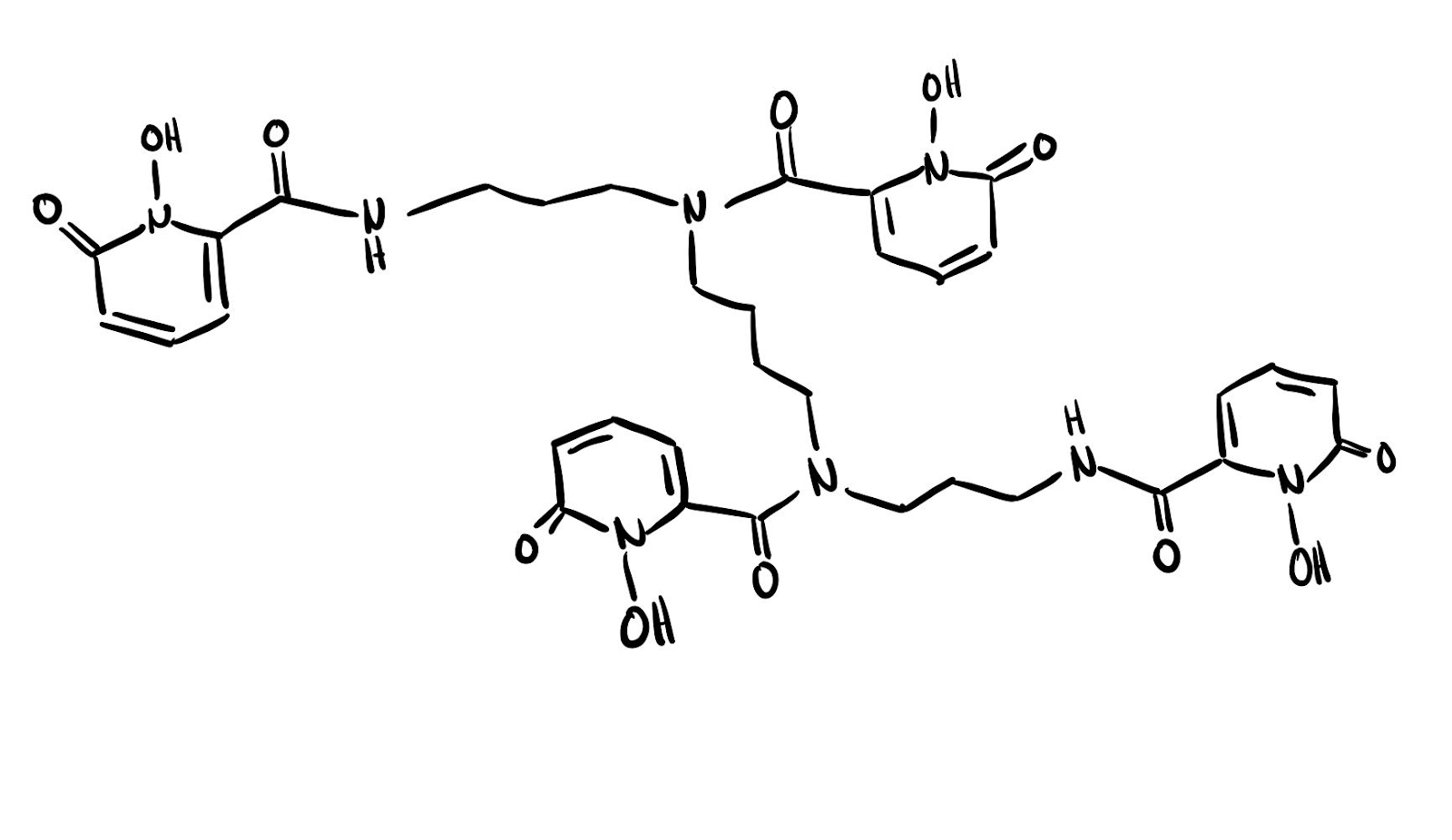
Figure 1. 3,4,3-LI(1,2-HOPO) ligand with a carbon and nitrogen backbone and eight binding oxygens.
The HOPO ligand consists of a backbone of carbon and nitrogen and eight oxygen binding sites. HOPO has been shown to have a high binding affinity to other similar heavy elements to einsteinium while also maintaining stability that makes it easier to study the luminescence spectrum of these elements.5 This stability is critical when working with such small quantities of the sample as in this case.
Several techniques were used to study the einsteinium-HOPO complex. The first is luminescence sensitization spectroscopy, which enables the investigation of electronic transitions in a material over a range of wavelengths. In this case, it was used to determine that the coordination complex contained einsteinium in the +3 state.3 X-ray absorption spectroscopy was also used to investigate the complex. This technique uses high-powered X-ray beams from a synchrotron to reveal the electronic structure of a compound.3 The team from Berkeley was able to use X-ray absorption spectroscopy to calculate the L3-metal edge of the einsteinium. The metal edge is a measure of the energy that it takes to excite an electron into unfilled d orbitals. In this case, the energy was determined to be within 0.1% of the theoretically calculated value.3 These techniques were also used to determine that the bond distance between the metal and the ligand was approximately 2.38 Å.3
This study was important for several reasons. For one, the team discovered several novel properties of einsteinium, an important step forward for transplutonium chemistry. In addition, it demonstrated the viability of the HOPO ligand when used with einsteinium. In this study, the stability of this ligand enabled the study of minuscule quantities of einsteinium, opening the door for use with other of einsteinium’s transplutonium relatives. Although einsteinium’s uses are likely to remain confined to the scientific and research-oriented worlds, the techniques pioneered could help investigate other transplutonium elements with more practical uses. For instance, companies such as Actinium Pharmaceuticals are using Actinium-225 in an attempt to find new and more effective cancer treatments.6
References
- Haire, R.G. (2008). Einsteinium. In: Morss, L.R., Edelstein, N.M., Fuger, J. (eds) The Chemistry of the Actinide and Transactinide Elements. Springer, Dordrecht. https://doi.org/10.1007/1-4020-3598-5_12
- Robinson, Sharon M., Benker, Dennis E., Collins, Emory D., Ezold, Julie G., Garrison, Jon R. and Hogle, Susan L.. “Production of Cf-252 and other transplutonium isotopes at Oak Ridge National Laboratory” Radiochimica Acta, vol. 108, no. 9, 2020, pp. 737-746. https://doi.org/10.1515/ract-2020-0008
- Carter, K.P., Shield, K.M., Smith, K.F. et al. Structural and spectroscopic characterization of an einsteinium complex. Nature 590, 85–88 (2021). https://doi.org/10.1038/s41586-020-03179-3
- Einsteinium Is Mysterious. Scientists Have Unlocked Some of Its Secrets. https://www.nytimes.com/2021/02/07/science/einsteinium-chemistry-elements.html. (Accessed 2022-04-21)
- Sturzbecher-Hoehne, M., Leung, C. N. P., D’Aléo, A., Kullgren, B., Prigent, A.-L., Shuh, D. K., Raymond, K. N., & Abergel, R. J. (2011). 3,4,3-LI(1,2-HOPO): In vitro formation of highly stable lanthanide complexes translates into efficacious in vivo europium decorporation. Dalton Transactions, 40(33), 8340–8346. https://doi.org/10.1039/C1DT10840A
- AWE Technologies. https://www.actiniumpharma.com/awe-technology. (accessed 2022-04-21)
