Lipid Nanoparticles, a New Direction for Genetic Vector Development
BY PATRICK TSENG
The standard method for transporting genetic material into cells is through viral vectors. For example, of the two gene therapies approved by the FDA (Luxturna and Zolgensma), both of them use viral vectors1. Only recently has the mention of lipid nanoparticles surfaced due to their implementation in mRNA vaccines made by Pfizer-BioNTech and Moderna2,3. And so, what are lipid nanoparticles, and how do they compare with viral vectors?
Viral vectors are bioengineered viral shells containing a cargo of DNA or RNA, which can then be transported into cells. Using viral envelopes was the obvious choice, since viruses have evolved to trick cells into taking them in. Examples of current pharmaceutical applications include some COVID-19 vaccines (Johnson & Johnson and AstraZeneca) and FDA approved drugs like Zolgensma1,4,5.
Lipid nanoparticles on the other hand, are made of lipids, which are a collection of molecules that have a long carbon chain with a functional group at the end. Lipids are what make up fats, e.g., animal fat or vegetable oil. The structure of a lipid nanoparticle is essentially a volume of water enveloped by a lipid bubble, which is usually in the form of a lipid bilayer, like what exists in cell membranes. Using this lipid shell, DNA or RNA residing in the enclosed volume of water can be transported into target cells6.
Between viral vectors and lipid nanoparticles, there are three key differences in immunogenicity, versatility, and synthesis. Immunogenicity is the creation of an immune response as a reaction to the introduction of foreign substances, whether they are viruses or lipid nanoparticles. If either kind of genetic vector causes an immune response, they become much less effective, since the immune system will eliminate the vectors before they can transport their DNA or RNA cargo. Unfortunately, as is the nature of a viral infection, the immune system will react to viruses, and the common symptoms of a viral infection will occur7. This would especially reduce the effectiveness of the viral vector over repeated uses. However, since a lipid nanoparticle is composed of only lipids, it mimics already existing lipid particles in blood and as such there is little to target by the immune system.6
As for the versatility of these two technologies, viral vectors are limited by the number of existing viruses that can be safely adapted for use as a viral vector. On the other hand, lipid nanoparticles can be modified by changing the composition of lipids, enabling easier and more diverse modifications that may confer useful attributes for delivering its DNA or mRNA cargo8. Below are two broad classes of lipids that are often incorporated into lipid nanoparticles and their chemical properties:
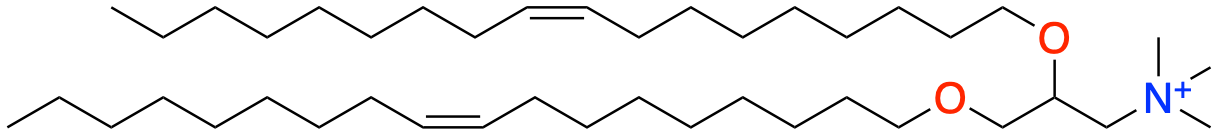
Cationic Lipids – Cationic Lipids are positively charged lipids that help form the nanoparticle around the mRNA. This works because of the attractive forces between the cationic lipid and the negative charges in the phosphate backbone of mRNA8. The DOTMA depicted above is an example of a commonly used cationic lipid. Note the nitrogen with a positive charge due to the four alkyl groups coming off of it, making the lipid cationic.
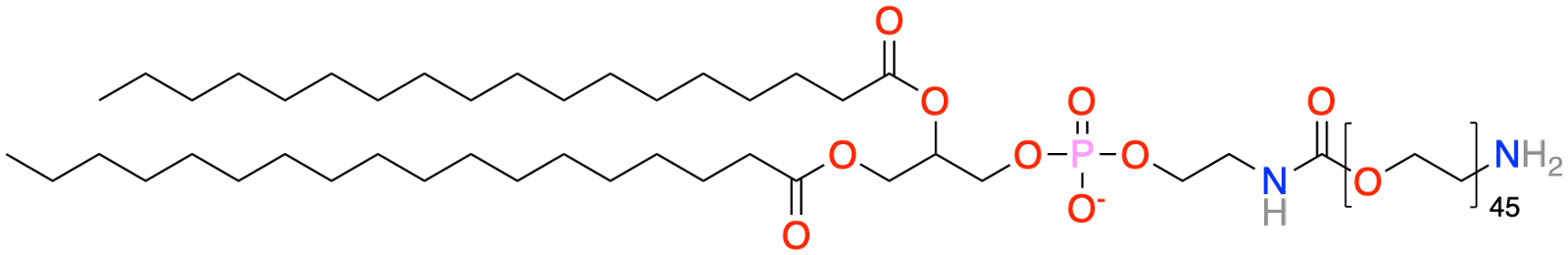
PEGylated Lipids – Polyethylene Glycol (PEG), a polymer with repeating units of [CH2-CH2-O], can be anchored onto a lipid and incorporated into the lipid nanoparticle. The polyethylene forms a layer of long hairs on the outer surface of the nanoparticle, shielding it from the environment. This increases the nanoparticle lifetime in circulation but unfortunately also reduces the chances of the nanoparticle entering the cell.8 The DSPE-PEG2000 depicted above is an example of a commonly used PEGylated Lipid. Note the region in brackets with the number 45, which is the PEG component of the molecule with 45 repeating units of the [CH2-CH2-O] motif.
Finally, there is a key difference in the method of synthesis between viral vectors and lipid nanoparticles. Viral vectors are made by infecting a cell with a bioengineered virus and adjusting the genetic code the vector placed into the cell to deliver a DNA or RNA cargo. The infected cell then begins to produce viral vectors as long as the cell is alive9. On the other hand, lipid nanoparticles can be made by forcefully mixing lipids, DNA/RNA cargo, and water to allow for proper self-assembly of the lipid nanoparticles. Comparing these methods, making lipid nanoparticles is much easier6.
Overall, lipid nanoparticles have almost no downsides in comparison to viral vectors. Much more research needs to be done to expand on and improve the functionalization of these lipid nanoparticles for more controllable and effective targeting to improve their adaptability as a genetic vector. While still a relatively new technology, lipid nanoparticles provide a promising new vector that delivers DNA or RNA that may overtake the applications of viral vectors.
References
- “Approved Cellular and Gene Therapy Products.” U.S. Food and Drug Administration, FDA, 26 Oct. 2021, https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products.
- United States, Food and Drug Administration. MRNA-1273 SPONSOR BRIEFING DOCUMENT. Food and Drug Administration, 17 Dec. 2020.
- United States, Food and Drug Administration. PFIZER-BIONTECH COVID-19 VACCINE (BNT162, PF-07302048) VACCINES AND RELATED BIOLOGICAL PRODUCTS ADVISORY COMMITTEE BRIEFING DOCUMENT. Food and Drug Administration, 30 Nov. 2020.
- European Union, European Medicines Agency. COVID-19 Vaccine AstraZeneca Common name: COVID-19 Vaccine (ChAdOx1-S [recombinant]). European Medicines Agency, 29 Jan. 2021.
- United States, Food and Drug Administration. COVID-19 Vaccine Ad26.COV2.S VAC31518 (JNJ-78436735) SPONSOR BRIEFING DOCUMENT. Food and Drug Administration, 26 Feb. 2021.
- Reichmuth, Andreas M, et al. “MRNA Vaccine Delivery Using Lipid Nanoparticles.” Therapeutic Delivery, vol. 7, no. 5, 14 Apr. 2016, pp. 319–334., https://doi.org/10.4155/tde-2016-0006.
- Bessis, N, et al. “Immune Responses to Gene Therapy Vectors: Influence on Vector Function and Effector Mechanisms.” Gene Therapy, vol. 11, no. S1, 2004, https://doi.org/10.1038/sj.gt.3302364.
- Kulkarni, Jayesh A., et al. “On the Role of Helper Lipids in Lipid Nanoparticle Formulations of Sirna.” Nanoscale, vol. 11, no. 45, 21 Nov. 2019, pp. 21733–21739., https://doi.org/10.1039/c9nr09347h.
- Kimura, Toyokazu, et al. “Production of Adeno-Associated Virus Vectors for in Vitro and in Vivo Applications.” Scientific Reports, vol. 9, no. 1, 2019, https://doi.org/10.1038/s41598-019-49624-w.
